Covalent Lewis Dot Structure Calculator
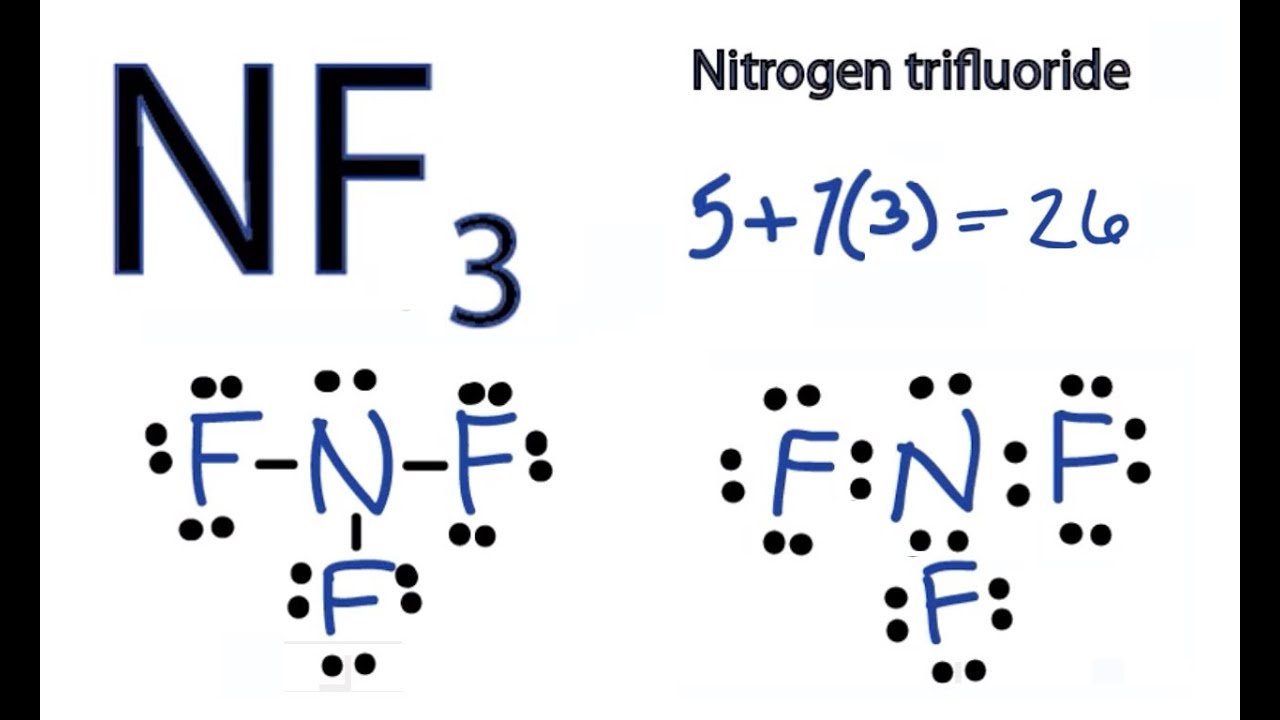
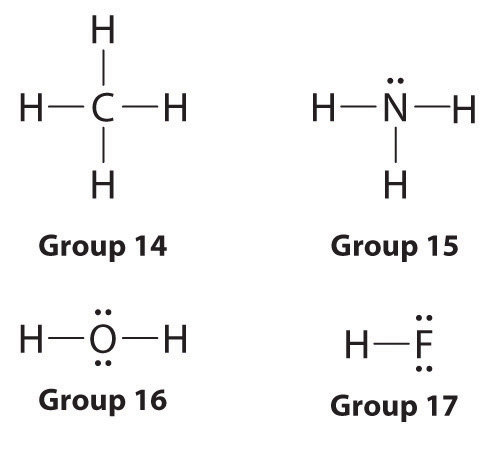
Covalent bonds share electrons in order to form a stable octet around each atom in the molecules. Hydrogen is the exception it only requires 2 electrons (a duet) to be stable. How do we draw a covalent Lewis Dot Structure? Level 1 (basic) 1. Add up all the valance electrons of the atoms involved. In the structure below, fluorine has six electrons in three lone pairs (not drawn) and the seventh electron participating in a covalent single bond to the Carbon, which allows the Fluorine to have an octet. Likewise, the carbon achieves its octet by sharing bonds with the two F and two H allows. Hope this helps. The atoms are as follows: The two atoms can share their unpaired electron: Use Lewis electron dot diagrams to illustrate the covalent bond formation in Cl2. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Get the free 'Lewis structure' widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram Alpha.
Mechanical fuel pump pressure regulator
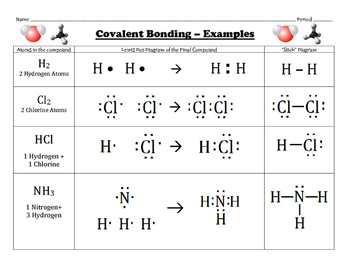
Lewis Dot Structure Finder
Lewis Dot Structure Calculator With Dots
Lewis Dot Structure for Boron Oxide (B2O3) Boron (metal) has 3 valence electrons Oxygen (nonmetal) has 6 valence electrons, needs 2 to be balanced B3↘ ↙O2 B2 O3 Lewis Dot Structure for Sodium Fluoride Na +1 Cl-1 Na has 1 valence electron Fl needs 1 to be balanced Na Cl Covalent Bonds Covalent Bonds are attractions of 2 nonmetals They have low melting/boiling points and aren't a good .. BCl3. Back. Windows 10 sound set default greyed out. 70 More Lewis Dot Structures. Daytona championship usa download. Note**Boron is an exception and will not form an octet. Hwk ufs micro saras soft setup driver for mac. Contains 3 bonding pairs and no lone pairs. This correlates with the property that it is dangerously reactive. Note- BF3,BBr3,BI3 are the same shapes.