Chemistry Lewis Dot Structure Calculator
Lewis Electron Dot Structure Calculator. What is the lewis dot structure for ozone? Chemistry stack exchange electron (lewis) structures pages 1 4 flip pdf download fliphtml5 why hcooh written like bottom but not top? Doesn t top satisfy octet rule?: chemhelp formation of kcl with an structure? Quora diagram chemical bonds full version hd quality diagramtonyb nowroma it. Get the free 'Lewis Structure Finder' widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram Alpha. Get the free 'Lewis structure' widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram Alpha.
Learning Objectives
By the end of this section, you will be able to:
Lewis structure calculator, This page lets you easily convert IUPAC names, common names, SMILES codes, CAS numbers, and other identifiers into chemical structures. On the back end it employs OpenChemLib to decode SMILES codes, the OPSIN library developed by Daniel Lowe, data from PubChem, various drug and natural product dictionaries, and openmolecules software.
- Write Lewis symbols for neutral atoms and ions
Lewis Symbols of Monoatomic Elements
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does not matter what order the positions are used.)
For example, the Lewis electron dot diagram for calcium is simply
Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table.
Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur:Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds.Figure 2. Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. The total number of electrons does not change.
Example 1: Writing Lewis DoT SYmbols of Elements
What is the Lewis electron dot diagram for each element?
- aluminum
- selenium
The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons (or three single dots around the atom):
The valence electron configuration for selenium is 4s24p4. In the highest-numbered shell, the n = 4 shell, there are six electrons. Its electron dot diagram is as follows:
Check Your Learning
What is the Lewis electron dot diagram for each element?
- phosphorus
- argon
Example 2: Writing Lewis DoT SYmbols of Ions
What is the Lewis electron dot diagram for each ion?
- Ca2+
- O2−
Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca2+.
Ca2+
The O2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows:
Check Your Learning

The valence electron configuration of thallium, whose symbol is Tl, is 6s25d106p1. What is the Lewis electron dot diagram for the Tl+ ion?
Key Takeaways
- Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol.
- Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom.
Exercises
1. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
2. Is it necessary for the first dot around an atomic symbol to go on a particular side of the atomic symbol?
3. What column of the periodic table has Lewis electron dot diagrams with two electrons?
4. What column of the periodic table has Lewis electron dot diagrams that have six electrons in them?
5. Draw the Lewis electron dot diagram for each element.
a) strontium
b) silicon
6. Draw the Lewis electron dot diagram for each element.
a) krypton
b) sulfur
7. Draw the Lewis electron dot diagram for each element.
a) titanium
b) phosphorus
8. Draw the Lewis electron dot diagram for each element.
a) bromine
b) gallium
9. Draw the Lewis electron dot diagram for each ion.
a) Mg2+
b) S2−
10. Draw the Lewis electron dot diagram for each ion.
a) In+
b) Br−
11. Draw the Lewis electron dot diagram for each ion.
a) Fe2+
b) N3−
12. Draw the Lewis electron dot diagram for each ion.
a) H+
b) H−
Show Select Answer1. The first two electrons in a valence shell are s electrons, which are paired.

3. The second column of the periodic table
5.
a)
b)
7.
a)
b)
9.
a) Mg2+
:max_bytes(150000):strip_icc()/Lewis-dot-58f78f405f9b581d5938e617.jpg)
b)
11.
Chemistry Lewis Dot Structure Calculator Worksheet
a) Fe2+
b)
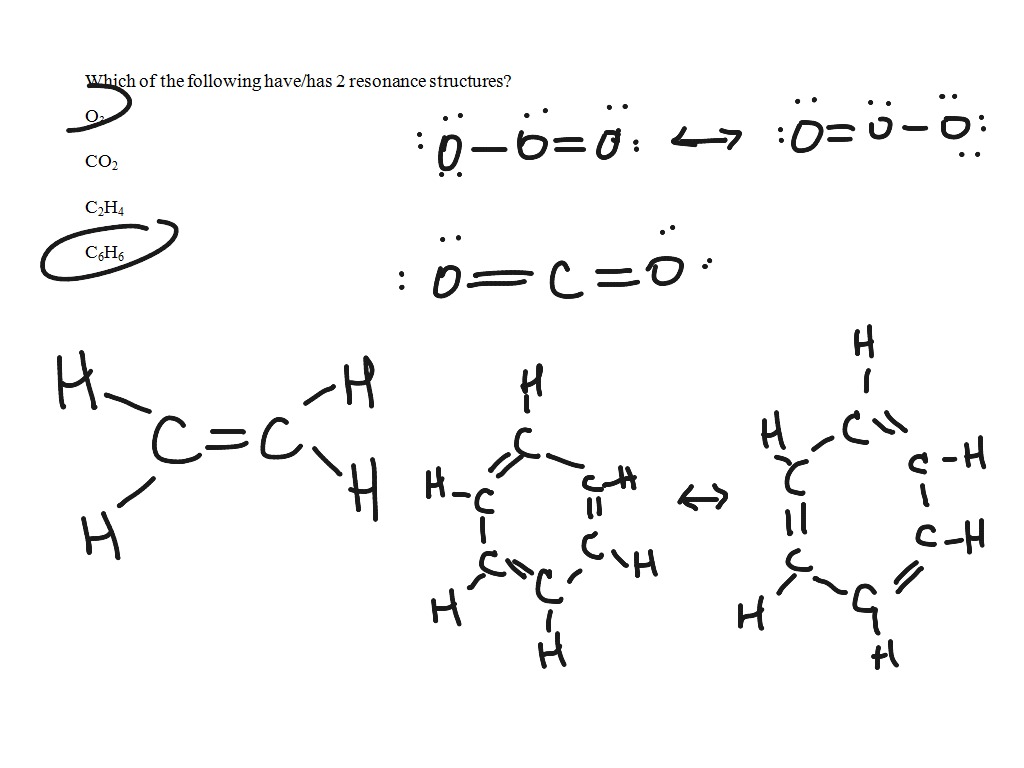
Lewis Dot Structures
A bond is the sharing of 2 electrons. Covalent bonds share electrons in order to form a stable octet around each atom in the molecules. Hydrogen is the exception it only requires 2 electrons (a duet) to be stable. How do we draw a covalent Lewis Dot Structure? Level 1 (basic) 1. Add up all the valance electrons of the atoms involved. ex CF4 So C has 4 and F has 7 (x4 we have 4Fs) = 32 valence electrons 2. You need to pick the central atom. This is usually easy, this atom will be surrounded by the others. Never H. So C will be surrounded by F's. 3. Now we create our skeleton structure by placing bonds in. A bond is a dash that represents 2 electrons. We have now placed 8 electrons as 4 bonds. We have 32-8= 24 more to place. 4. Starting with the outer atoms add the remaining electrons in pairs until all the electrons have run out.
All 32 electrons are now in place, count the dots around each F. 6 dots and a bond (2 electrons) is 8. We have our octet. The carbon has 4 bonds (2electrons) for its 8. DONE Level 2 (Double and Triple bonds) Same rules apply until #4 1. Add up all the valance electrons of the atoms involved. ex CO2 So C has 4 and O has 6 (x2 ) = 16 valence electrons 2. You need to pick the central atom. This is usually easy, this atom will be surrounded by the others. Never H. So C will be surrounded by O's. 3. Now we create our skeleton structure by placing bonds in. A bond is a dash that represents 2 electrons. We have now placed 4 electrons as 2 bonds. We have 16-4=12 more to place. 4. Starting with the outer atoms add the remaining electrons in pairs until all the electrons have run out.
All 16 electrons are now in place, count the dots around each O. 6 dots and a bond (2 electrons) is 8. We have our octet. The carbon has 2 bonds (2electrons) for its 4....? We need 8, so move a pair of electrons from the O to between the C and O. It will share 2 pairs of electrons instead of 1. It now has a double bond instead of a single bond.
now they all have an octet, it cleans up like this Make it symmetrical. Level 3-Lewis Dots of Polyatomic Ions Same rules apply, at the end they get brackets and a charge AP Chemistry and or College Level Rules 1. Determine whether the compound is covalent or ionic. If covalent, treat the entire molecule. If ionic, treat each ion separately. Compounds of low electronegativity metals with high electronegativity nonmetals (DEN > 1.7) are ionic as are compounds of metals with polyatomic anions. For a monoatomic ion, the electronic configuration of the ion represents the correct Lewis structure. For compounds containing complex ions, you must learn to recognize the formulas of cations and anions. 2. Determine the total number of valence electrons available to the molecule or ion by:
3. Organize the atoms so there is a central atom (usually the least electronegative) surrounded by ligand (outer) atoms. Hydrogen is never the central atom. 4. Determine a provisional electron distribution by arranging the electron pairs (E.P.) in the following manner until all available pairs have been distributed:
5. Calculate the formal charge (F) on the central atom.
6. If the central atom formal charge is zero or is equal to the charge on the species, the provisional electron distribution from (4) is correct. Calculate the formal charge of the ligand atoms to complete the Lewis structure. 7. If the structure is not correct, calculate the formal charge on each of the ligand atoms. Then to obtain the correct structure, form a multiple bond by sharing an electron pair from the ligand atom that has the most negative formal charge.
8. Recalculate the formal charge of each atom to complete the Lewis structure. on to Formal Charge Chemical Demonstration Videos |